INTRODUCTION: Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative therapy for numerous malignant and non-malignant hematologic diseases. To enable engraftment of donor hematopoietic stem cells (HSCs), recipients undergo conditioning with chemotherapy and irradiation to make marrow space for incoming donor HSCs and suppress host immune responses that may lead to graft rejection. These cytotoxic conditioning regimens carry risks of severe toxicities which prevents many patients from accessing the lifesaving benefit of allo-HSCT. Previously, we developed fully ablative CD45- and cKit-targeted antibody-drug conjugates (ADC) which, when combined with Janus kinase 1/2 (JAK1/2) inhibitors, enabled fully mismatched allo-HSCT with full donor chimerism and minimal toxicity while providing durable antileukemia activity (Persaud et al (2022), ASH Abstract). However, even ADCs carry potential risks of acute and chronic toxicities that may preclude their common use as HSCT conditioning agents. Moreover, unlike malignant diseases, allo-HSCT for non-malignant diseases does not require the conditioning regimen to provide antitumor benefit and partial donor chimerism may be sufficient for cure, strongly favoring the use of less intense conditioning strategies with the fewest possible toxicities. We hypothesized that naked antibody-based conditioning strategies can achieve the therapeutic benefits of allo-HSCT for non-malignant hematologic diseases, obviating exposure to toxic ADC payloads.
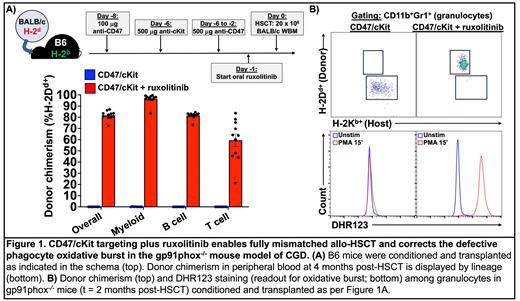
METHODS: We combined unconjugated antibodies targeting CD47 and cKit with JAK1/2 inhibition to perform minor histocompatibility antigen (miHA)-mismatched (129/Sv→B6-Ly5.1) and fully mismatched (BALB/c→B6) murine allo-HSCT. In some full mismatch HSCT experiments, recipients included B6-gp91phox -/- mice, which serve as a model of chronic granulomatous disease (CGD) due to their deficiency in a catalytic subunit of NADPH oxidase. Mice received 100 µg anti-CD47 (clone mIAP410; BioXcell) 8 days before HSCT (d-8) as a priming dose to reduce the severity of CD47 blockade-induced anemia. Next, mice received 500 µg anti-cKit (clone ACK2; BioLegend) on d-6 and daily 500 µg doses of anti-CD47 from d-6 through d-2. For JAK1/2 inhibition, mice received oral ruxolitinib (Incyte; 2 mg drug/g chow) starting d-3 or d-1 and continuing until d+28. On d0, recipients were transplanted with 20 x 10 6 whole bone marrow cells from 129/Sv (miHA) or BALB/c (fully mismatched) mice. Peripheral blood donor chimerism was followed monthly by flow cytometry for CD45.2 + (miHA) or H-2D d+ (fully mismatched) cells. Oxidative burst among granulocytes in B6-gp91phox -/- recipients was assessed by dihydrorhodamine 123 (DHR123) staining after phorbol 12-myristate 13-acetate (PMA) stimulation.
RESULTS: CD47 and cKit antibodies plus ruxolitinib enabled multilineage engraftment in both miHA and fully mismatched allo-HSCT, including myeloid lineage donor chimerism of 95-99% (Figure 1A). As expected, CD47 blockade induced significant but transient morbidity from anemia during the peritransplant period; notably, a shorter duration of ruxolitinib treatment before HSCT (starting d-1 instead of d-3) improved the tolerability of the regimen without impacting engraftment success. Allo-HSCT of B6-gp91phox -/- mice led to near complete replacement of host granulocytes in peripheral blood with donor granulocytes, whose DHR123 positivity upon PMA stimulation indicated an intact oxidative burst capacity (Figure 1B).
CONCLUSIONS: Combination of antibodies targeting CD47 and cKit with JAK1/2 inhibition provides a novel conditioning strategy for allo-HSCT in mouse models, achieving high-level, stable multilineage engraftment without radiation, chemotherapy, or exposure to toxic ADC payloads. The near complete donor chimerism among granulocytes made it particularly well suited to correcting phagocyte oxidase function in the gp91phox -/- model of CGD. Future studies seek to 1) mitigate the peritransplant anemia caused by CD47 blockade using Fc-silenced anti-CD47 reagents, 2) determine the proportions of donor- versus recipient-derived myeloid cells in non-hematopoietic tissues of allo-HSCT recipients, and 3) use murine infection models to evaluate the impact of allo-HSCT on host defense in transplanted B6-gp91phox -/- recipients.
Disclosures
DiPersio:RiverVest Venture Partners: Membership on an entity's Board of Directors or advisory committees; BiolineRx Ltd: Consultancy, Research Funding; WUGEN: Other: Ownership Investment, Patents & Royalties; Incyte: Consultancy, Research Funding; NeoImmune Tech: Consultancy; Macrogenics: Consultancy, Research Funding; Washington University: Current Employment; Sun Pharma Ltd.: Membership on an entity's Board of Directors or advisory committees; Vertex: Consultancy; Magenta: Other: Ownership Investment, Patents & Royalties; Amphivena Therapeutics: Research Funding; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal